39 mock up labels and packages certification form
Packaging Design for Beginners: How to Create a Simple Box Go to File > Place, and choose a pattern from the Color splash patterns pack you downloaded earlier. Click Open, and allow the image to fill up the whole frame. Step 2 Use the Eyedropper Tool (I) to pick up the very pale pink color from the pattern. Double-click on the Fill Color box at the bottom of the Tools panel to open the Color Picker window. How to Create, Change, Fill colour in Pie Chart in R - EDUCBA pie(x, labels, radius, main, col, clockwise) Where, x is called a vector, and it contains the numeric values which are to be used in the pie chart, such as those production figures in the above example. labels take a name for those values in X, such as the name of chemicals. The radius argument is for the radius of the circle of the pie chart.
DOC CMDh_222_2007_Rev01_2011 07 - Heads of Medicines Agencies Provide the following: one full colour mock-up of the current approved label/PL, two full colour mock-ups of the proposed label/PL (one copy to be annotated with the proposed changes and one clean copy).
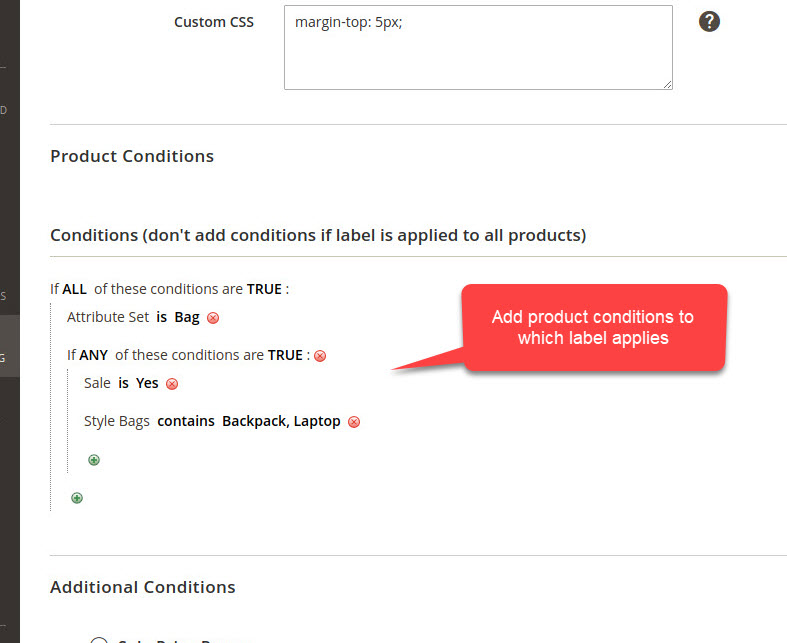
Mock up labels and packages certification form
PDF Investigational New Drug (IND) Submission checklist - FOI services Provide a copy of all labels and labeling for the investigational product. A mock-up or printed representation of the proposed labeling that will be provided to investigator(s) is acceptable. Investigational labels must carry a "caution" statement that reads: "Caution: New Drug - Limited by Federal (or United States) law to investigational use ... Chemistry, Manufacturing, and Control Information for Human Gene ... New Drug Applications (INDs) Guidance for Industry Additional copies of this guidance are available from the Office of Communication, Outreach and Development (OCOD), 10903 New Hampshire Ave.,... PDF Approval Package for - Food and Drug Administration draft or mock-up form, two copies of both the promotional materials and package insert directly to: Food and Drug Administration Center for Drug Evaluation and Research Division of Drug Marketing, Advertising, and Communications 5901-B Ammendale Road Beltsville, MD 20705 We call your attention to 21 CFR 314.81(b)(3) which requires
Mock up labels and packages certification form. TENDER ADDENDUM NOTICE NUMBER 1 BMorningtonb Shire Council - US Legal Forms Open it up with online editor and start altering. Fill out the blank fields; engaged parties names, places of residence and phone numbers etc. Change the template with exclusive fillable areas. Include the day/time and place your e-signature. Click on Done after double-examining all the data. Changing the labelling and package leaflet (Article 61(3) notifications ... Any supportive relevant documentation [e.g.: User Testing reports English and multi-lingual ('worst-case') colour mock-up of outer and immediate packaging for each pharmaceutical form in each container type (e.g. blister and bottle, vial and pen) in the smallest pack-size] to the 61(3) Notification, presented under the appropriate headings and ... Custom Packaging Design | Package Printing | Vaughan | Toronto ... We are a professional packaging design company in Vaughan offering custom package printing services. Serving clients in Greater Toronto and surrounding Area. ... Digital Printing; Packaging Printing; Label Printing; Banner Printing; Packaging; Labels; Banners; Graphic Design; Request Quote; Download Template; About Us; Contact Us; Call: 905-850 ... Traco Packaging - Custom Labels and Shrink Sleeves As with any custom flexible packaging company, the novel Coronavirus and Covid-19 has changed the way we work, and we are working hard to mitigate its effects on our business. Some actions we are currently taking in an effort to control lead times and deliver what we promise: Investing in more equipment. Hiring aggressively. Creating extra shifts.
Specimens | USPTO Is a digitally created or altered image or mock-up; Is material used only to conduct your daily business of selling goods (e.g., packing slips, business stationery, order forms, waybills, and bills of lading) Is only a drawing or depiction of your trademark; Is a webpage that doesn't include the URL and date accessed/printed Guidance Document: Administrative Processing of Submissions … 01/10/2020 · a Labels and Packages Certification Form stating that the label and packaging material are similar to the original product with respect to size and placement of graphics, logos and font, as applicable ... Due to the regulatory mock-up requirements under the Regulations Amending the Food and Drug Regulations (Labelling, Packaging and Brand Names ... 25 Best Packaging Design Courses Online (Free & Paid) - JUST™ Creative Product Packaging Design And Mockup: Creative Level — Best for Mockups All 25 packaging design courses listed below Free Graphic Design Software Trial + Adobe CC Discount If you're going to become a professional package designer, a subscription to the industry standard software, Adobe Creative Cloud is recommended. PDF Labels and Packages Certification Form for Prescription Products A mock-up of the Package Insert is enclosed in both official languages. Any necessary clarifications have been provided within a Note to Reviewer in Module 1.3.2. The first language Package Insert mock-up has been provided and the second language Package Insert mock-up will be provided within 20 days of the submission being accepted into review.
PDF Labels and Packages Certification Form for Non-prescription Drugs and ... A mock-up of only the smallest label and/or package for each dosage form and strength has been provided in both official languages, as • there are no differences other than pill count or volume on the labels/packages; and • all the other labels/packages will have identical text, format, size, layout, color, etc. Forms: Applications and submissions for drug products 31/03/2003 · Labels and Packages Certification Form for Prescription Products [2020-12-21] Master File (MF) Application Fee Form for Human Drugs [in effect April 1 2022] (DOC Version - 55 KB) Non-prescription drug monograph attestation form (PDF fillable/saveable - 648 KB) [2016-01-15] Notice - Updated Screening Criteria for Generic Drug Submissions [2013 ... Compliance FAQs: Packaging and Labeling in the US | NIST There are many regulations, depending on the product, with which a product's label or markings must be in compliance before being sold in the United States. Labeling requirements related to legal metrology (i.e., products and commodities sold in package form by weight, measure or count) must comply with The Fair Packaging and Labeling Act ... Openssl ecdsa - kifmd.sirimiri.shop 15/02/2022 · Finally, Using cryptography 2 pem -out cert 04 + patches in Radiator goodies no longer work with the current version of EAP_52 To generate a Certificate Signing Request (CSR) using ECDSA to send to a public Certification Authority (CA) using Windows, open the local computer certificate store (certlm To generate a Certificate Signing Request (CSR). $ openssl …
Quality System Regulation Labeling Requirements | FDA All printed packaging and labeling materials, including preprinted containers, inserts and preprinted packaging materials, must be stored in an area and manner suitable to prevent mixups ( 21 CFR...
Inspection Forms | Food Safety and Inspection Service Listed below are the official forms available for downloading or printing. Only official forms are acceptable in the conduct of Agency business, so please carefully read the information and instructions pertaining to the form (s) you wish to use. Some of our forms have fill-and-print capability; others will have save-and-send capability.
PDF Labels and Packages Certification Form for Prescription Products Labels and Packages Certification Form for Prescription Products Author: Government of Canada - Health Canada Subject: Labels and Packages Certification Form for Prescription Products Created Date: 12/2/2020 10:52:32 AM
List of R Packages | Complete Guide to the Top 16 R Packages Conclusion-List of R Packages. There are numerous packages in R, and the application of a package depends on requirements. The List of R Packages community has been growing very fast, and every single day a package gets added. Multiple packages may provide similar functionalities but the selection of a package must be based on its careful study.
Comprehensive Guide to DALL-E By OpenAI: Creating Images from Text 02/03/2021 · Stage 2 : Concatenate up to 256 BPE-encoded text tokens with the 32 × 32 = 1024 image tokens, and train an autoregressive transformer to model the joint distribution over the text and image tokens. Note : The details of DVAE are given in the Appendix of Research Paper . Apart from generating images from scratch, the above approach helps to reproduce a …
Data Preprocessing With R: Hands-On Tutorial - Analytics India … 15/01/2019 · When it comes to Machine Learning and Artificial intelligence there are only a few top-performing programming languages to choose from. In the previous tutorial, we learned how to do Data Preprocessing in Python.Since R is among the top performers in Data Science, in this tutorial we will learn to perform Data Preprocessing task with R.
PDF Buy the Book >> Chapter 10 Plain Language Labeling (PLL) - RAPS methods, mock-up preparation and a continuous improvement approach. Factors to consider include type style, weight and size; use of TALL man lettering (writing groups in ... • Labels and Packages Certification Form for Nonprescription Drugs Certification forms are included at fil-ing and certify such items as the trans-lation fidelity ...
Forms | FDA - U.S. Food and Drug Administration Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Use the following instructions to download the form if you encounter an issue:
Free Medical Form Templates | Smartsheet Download Medical Chart Template. Excel | Word | PDF. Doctors and health service providers can use this downloadable template to document a patient's medical details during an appointment, from initial exam to progress notes. This template includes space to detail everything from main medical concerns to reason for visit, family and medical ...
DOCX Mock-Up Label s and Packages Certification Form for Non-p rescription Drugs and Contact Lens Disinfectants Protected A DRUG PRODUCT INFORMATION Submission Type Brand, Proprietary or Product Name (as per Field #8 on the Drug Submission Application Form)
Developer Portal | Salesforce Developers In Salesforce’s enhanced CMS, Sidebar Extensions put productivity tools right inside the content editor where your content creators need them. Sidebar extensions let you add useful features like spell checkers, grammar and tone editors, content recommendation apps, translation services … and anything else you can dream up. August 29, 2022
Mock-Up-Guidance Document - BASG Certification, authorisation, and inspection of tissue establishments Medical Device Operators ... Forms for medicinal products Change of ownership acc. to Art 25 AMG ... Mock-Up-Guidance Document messages in brief | 19/11/2018 ...








Post a Comment for "39 mock up labels and packages certification form"